Laekna Announces Robust Phase 1b data of LAE002 (afuresertib) for treatment of HR+/ HER2- Breast Cancer at ESMO Congress 2024
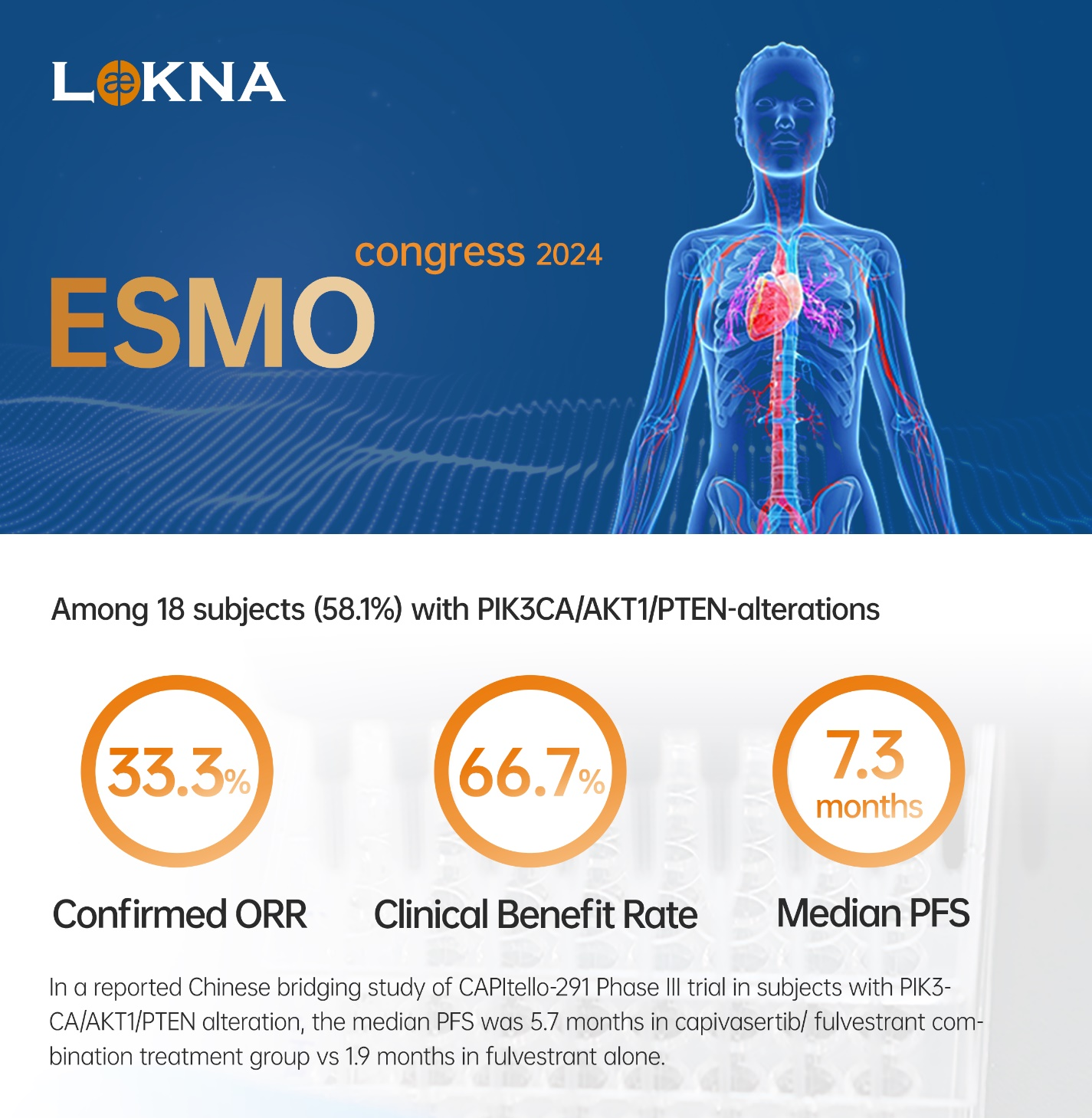
- 58.1% of subjects with PIK3CA/AKT1/PTEN alteration and 64.5% with prior CDK4/6 inhibitors
- Confirmed ORR was 33.3%, CBR was 66.7%, and the median PFS was 7.3 months
- In a reported Chinese bridging study of CAPItello-291 Phase III trial in subjects with PIK3CA/AKT1/PTEN alteration, the median PFS was 5.7 months in capivasertib/ fulvestrant combination treatment vs 1.9 months in fulvestrant alone
- Phase III clinical trial AFFIRM-205 led by Academician Binghe Xu is moving smoothly, ahead of the planned schedule
September 19, 2024 — Laekna, Inc. (2105.HK) today announced that the end of study results of its phase Ib clinical trial of LAE002 (afuresertib, an oral AKT inhibitor) plus fulvestrant (the “Combination Therapy”) in subjects with locally advanced or metastatic HR+/HER2- breast cancer (“LA/mBC “) who failed standard of care therapies were presented at the European Society for Medical Oncology (ESMO) Congress 2024.
This phase Ib clinical trial is a single arm, open-label study to evaluate the efficacy and safety of the Combination Therapy in subjects with HR+/HER2- LA/mBC who have disease progression after 1–2 prior lines of endocrine therapy with or without a CDK4/6 inhibitor (≤1 line), and/or ≤1 line of chemotherapy.
As of the data cut-off date of August 27, 2024, 31 subjects were enrolled, including 28 Chinese subjects and 3 American subjects. The median age of all subjects was 54 years old. 71.0% of the subjects had received one line of therapy, and 29.0% had received two lines of therapy. 18 of subjects (58.1%) had PIK3CA/AKT1/PTEN alteration. 64.5% of the subjects were previously treated with CDK4/6 inhibitors. The median duration of follow-up was 17.3 months. Results of the Phase Ib study in all 31 subjects and in a subgroup with PIK3CA/AKT1/PTEN alterations from U.S. and China have demonstrated promising anti-cancer efficacy with a well-tolerated safety profile.
Laekna has thus commenced a subsequent phase III clinical trial AFFIRM-205 led by Academician Binghe Xu, MD, PhD, the Cancer Hospital Chinese Academy of Medical Science, in China for the Combination Therapy in patients with PIK3CA/AKT1/PTEN alterations in May 2024. The clinical trial is proceeding smoothly, ahead of the planned schedule.
“The results of our Phase Ib study in this Combination Therapy has shown promising efficacy and well-tolerated safety profile, especially among patients with PIK3CA/AKT1/PTEN alterations. It increased our confidence in expecting a positive outcome of AFFIRM-205 trial and a successful NDA submission. We are now advancing the Phase III clinical trial AFFIRM-205 in full speed at all sites. We are excited about the efficient progress and are committed to accelerate the progress to address the unmet medical needs of patients.,” said Dr. Chris LU, Chairman and Chief Executive Officer of Laekna.”
*ESMO Asia 2023 capivasertib+fulvestrant for patients with aromatase inhibitor-resistant HR-positive/HER2-negative advanced breast cancer: Phase3 CAPItello-291trial Chinese cohort
- End –
About LAE002 (afuresertib)
LAE002 (afuresertib) is one of the only two AKT inhibitors in late-stage development for breast and prostate cancer globally.
LAE002 (afuresertib) is a potent AKT inhibitor that inhibits all three AKT isoforms (AKT1, AKT2 and AKT3). LAE002 (afuresertib) has demonstrated several advantages compared to other AKT inhibitors, including higher efficacy, better potency, more significant tumor inhibition exposure and a better safety profile, based on public data. Capivasertib is the first approved AKT inhibitor from AstraZeneca, which FDA approved for HR+/HER2- breast cancer in November 2023.
Our experience in executing and developing combination therapies among our pipeline has well demonstrated our ability to unleash the clinical value of our pipeline products. Our LAE002 (afuresertib) combination trial with Fulvestrant has demonstrated great clinical value to treat HR+/HER2- breast cancer patients who have failed prior standard care treatments of endocrine/anti-estrogen therapies including CDK4/6 inhibitors, a big unmet medical need with huge market potential.
About HR+/HER2- Breast Cancer
The latest data released by the International Agency for Research on Cancer (IARC) of the World Health Organization indicated that the estimated number of new cases of breast cancer in 2022 reached 2.29 million worldwide, ranked as the most frequent cancer in females with 666,103 deaths. The IARC also reported that breast cancer ranked second among women in China, with an estimated number of 357,161 new cases in 2022.
Approximately 69% of breast cancer patients in the United States were found to be HR+/HER2-, and the proportion of this subtype among Chinese patients were approximately 62%. Although most of patients with this subtype of breast cancer can initially benefit from first/second-line treatment by endocrine therapy + CDK4/6 inhibitors and/or chemotherapy, they may gradually develop drug resistance and result in treatment failure. Novel treatment options are urgently needed for patients after drug resistance.
About Laekna
Stock Code: 2105.HK
Founded in 2016, Laekna is a science-driven, clinical-stage biotechnology company committed to bringing novel therapies to patients with cancer, metabolic diseases and liver fibrosis patients around the world.
As of June 30, 2024, Laekna has initiated seven clinical trials for LAE102, LAE002 (afuresertib), LAE001 and LAE005 to address unmet medical needs in obesity and cancers.
LAE102 is our internally discovered antibody against ActRIIA. It has been shown in the pre-clinical studies to increase lean mass and decrease fat mass. We’ve obtained IND approvals from the FDA and the CDE for LAE102 in obesity indication and are advancing the Phase I clinical trial in China.
Blocking Activin-ActRII pathway could promote muscle regeneration and decrease fat mass. Laekna team has accumulated tremendous experiences and deep know-how in this specific field and is developing more drug candidates (LAE103 and LAE123), in addition to LAE102, to maximize the value of targeting ActRII receptors.
In the cancer area, Laekna has built a comprehensive portfolio of drug candidates, covering the treatment of breast cancer, prostate cancer, ovarian cancer and PD-1/ PD-L1 drug-resistant solid tumors. LAE002 (afuresertib) is a potent AKT inhibitor that inhibits all three AKT isoforms (AKT1, AKT2 and AKT3) as well as one of the only two AKT inhibitors in late-stage development for breast and prostate cancer globally. Laekna has commenced the Phase III clinical trial (AFFIRM-205) for LAE002 in patients with HR+/HER2- breast cancer.
Laekna, Inc. (2105.HK) was listed on the Main Board of The Stock Exchange of Hong Kong Limited (the “Hong Kong Stock Exchange”) on June 29, 2023.
For more information, please visit: https://www.laekna.com/ or https://www.linkedin.com/company/74110713/
1 Global Cancer. IARC. http://gco.iaic.fr/
2 Global Cancer. IARC. http://gco.iaic.fr/
3 Cancer Stat Facts: Female Breast Cancer Subtypes; SEER 22 2016–2020
4 Breast cancer subtypes and survival in Chinese women with operable primary breast cancer. Chin J Cancer Res, 2011. 23(2): p. 134-9.
Forward Looking Statements
This press release may contain certain “forward-looking statements” which are not historical facts, but instead are predictions about future events based on Laekna’s current beliefs, assumptions, and expectations, commonly identified by words such as "would", "may", "expects", "believes", "plans", "intends", "projects" and other terms with similar meaning. Although we believe that our predictions are reasonable, future events are inherently uncertain and our actual future results or performance may be materially different from what we expect. Accordingly, you are strongly cautioned that reliance on any forward-looking statements is subject to significant known and unknown risks and uncertainties. All forward-looking statements contained herein are qualified by reference to the cautionary statements set forth in this section. All information provided in this press release is as of the date of this press release and are based on assumptions that we believe to be reasonable as of this date, and we do not undertake any obligation to update any forward-looking statement, except as required under applicable law.
Contact us:
Media communication@laekna.com
Business Development bd@laekna.com
-
 2024-11-20Laekna Announces a Clinical Collaboration with Lilly to Develop LAE102, a Novel Monoclonal Antibody Targeting Activin Receptor Type 2A for The Treatment of ObesityMore
2024-11-20Laekna Announces a Clinical Collaboration with Lilly to Develop LAE102, a Novel Monoclonal Antibody Targeting Activin Receptor Type 2A for The Treatment of ObesityMore -
 2024-06-26Laekna Announces First Subject Dosed in Phase I Clinical Trial of LAE102 (a monoclonal antibody against ActRIIA) in China for The Treatment of ObesityMore
2024-06-26Laekna Announces First Subject Dosed in Phase I Clinical Trial of LAE102 (a monoclonal antibody against ActRIIA) in China for The Treatment of ObesityMore

Follow us on Linkedin







