Laekna (2105.HK) Announces Interim Results 2024
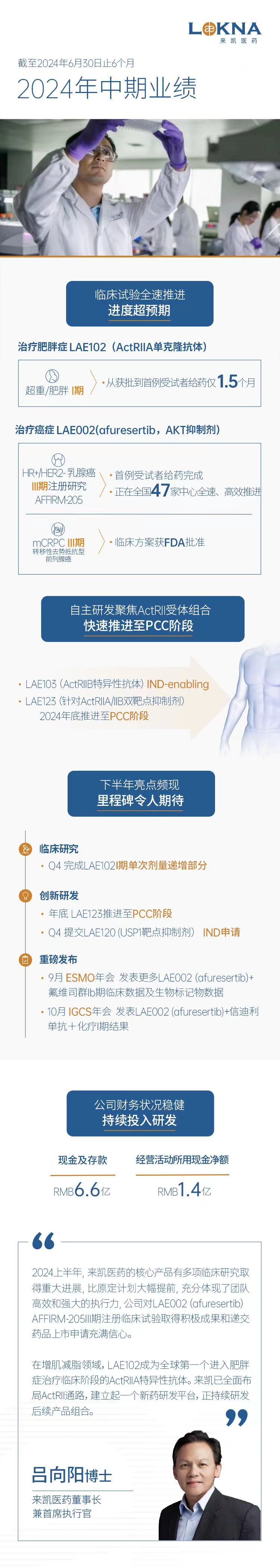
- Commenced the Phase I clinical study of LAE102, an internally discovered monoclonal antibody against ActRIIA, for the treatment of obesity and the first subject has been dosed, ahead of planned schedule
- Established a development platform of novel drugs targeting ActRII pathway, initiated IND-enabling study for LAE103, and targeted to advance LAE123 to PCC declaration by the end of 2024
- Accelerating the progress of AFFIRM-205, a Phase III clinical trial of LAE002 (afuresertib) plus fulvestrant for the treatment of HR+/HER2- breast cancer, in 47 sites in China
- Conference call of interim financial results will be hosted at 10:00 AM Beijing time on August 19, 2024 (Monday)
August 19, 2024 — Laekna, Inc. (2105.HK), a clinical-stage biotechnology company, announced the unaudited condensed consolidated interim results for the six months ended June 30, 2024, together with the highlights of the company’s innovative pipeline and progress achieved.
“We’ve made significant progress with respect to the clinical developments of our drug candidate assets and are ahead of our planned schedule. It demonstrated the remarkable efficiencies and strong execution abilities of Laekna team,” said Dr. Chris LU, Chairman and CEO of Laekna, Inc. “LAE102 is the first ActRIIA-specific antibody in clinical stage development for the treatment of obesity globally. It has been shown in the pre-clinical studies to be a potential drug candidate developed for quality weight management to increase lean mass and decrease fat mass. Laekna further expanded our comprehensive ActRII portfolio with LAE103 (ActRIIB-specific antibody) and LAE123(dual inhibitor against ActRIIA/IIB) and has established a novel drug development platform targeting ActRII pathway.”
Dr. Chirs LU also indicated strong confidences in LAE002 (afuresertib, our AKT inhibitor). He added: “The data of the Phase Ib study of LAE002 for the treatment of HR+/HER2- breast cancer has shown promising anti-cancer efficacy with a well-tolerated safety profile, especially in patients with PIK3CA/AKT1/PTEN alterations. It enhanced our confidence in expecting a positive outcome of our phase III clinical trial AFFIRM-205 and a successful NDA submission. Currently, we’re advancing the progress of AFFIRM-205 in 47 sites in China. We are committed to bringing this novel therapy to patients as soon as possible.”
Full speed accelerating the clinical studies and well ahead of planned schedule
In our ActRII portfolio, LAE102 is our internally discovered monoclonal antibody against ActRIIA. The company has obtained IND approvals from CDE and FDA in the second quarter of 2024. The phase I clinical study of LAE102 has been commenced in June 2024 and ahead of planned schedule.
This Phase I clinical study is a randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability and pharmacokinetics of the therapy. The company targets to achieve primary completion of the single ascending dose part (the SAD Study) of this Phase I clinical trial in the fourth quarter of 2024.
In our oncology portfolio, LAE002 (afuresertib), our core product, is a potent AKT inhibitor that inhibits all three AKT isoforms (AKT1, AKT2 and AKT3) as well as one of the two AKT inhibitors in late-stage development for breast and prostate cancer globally. LAE002 (afuresertib) has demonstrated several superior features compared to other AKT inhibitors, including higher efficacy, better potency, more significant tumor inhibition exposure and a better safety profile, based on the public data.
The company has made significant progress with respect to the clinical developments of this novel drug candidate and achieved following milestones:
- LAE002 (afuresertib)+Fulvestrant in HR+/HER2-breast cancer, Phase III, AFFIRM-205
The results of our Phase Ib study with 20 patients from the U.S. and China were presented during a poster spotlight session at the 2023 San Antonio Breast Cancer Symposium (SABCS) in December 2023. It has shown promising anti-cancer efficacy with a well-tolerated safety profile. The company enrolled 11 additional subjects in this Phase Ib study and further verified the promising anti-cancer efficacy with a well-tolerated safety profile indicated in the earlier stage of the study. The results of the study have been accepted by the European Society for Medical Oncology (ESMO) Congress in Barcelona, Spain in September 2024. The company will present the clinical data of all enrolled patients and the patients with positive biomarker in this Phase Ib study in a poster presentation.
Laekna has commenced the Phase III clinical trial AFFIRM-205 in China for LAE002 (afuresertib) plus fulvestrant in patients with PIK3CA/AKT1/PTEN alterations and HR+/HER2- locally advanced or metastatic breast cancer (LA/mBC) in May 2024, which was ahead of planned schedule. AFFIRM-205 is a multi-center, randomized, double-blind, placebo-controlled pivotal study to further assess the anti-tumor efficacy and safety of the combination therapy.
- LAE002 (afuresertib)+LAE001/prednisone in mCRPC, Phase II
As of November 21,2023, 40 patients who progressed on 1–3 lines of standard treatments, including at least 1 line of abiraterone, or the second generation of AR antagonists, had been enrolled in the recommended Phase II dose group. The median rPFS was 8.1 months. This is a significant improvement compared to the median rPFS of 2 to 4 months of mCRPC patients under the standard treatments historically. The combination therapy was generally tolerable with manageable treatment emergent adverse events and recoverable after routine treatments.
In May 2024, Laekna has obtained approval from FDA for the protocol of this Phase III clinical trial. The company plans to pursue strategic partnerships to accelerate the development and commercialization of LAE002 (afuresertib) and LAE001 to address the great unmet medical need for cancer therapies.
Focusing on internal discovery, advancing to PCC declaration
As of June 30, 2024, Laekna’s pre-clinical R&D team has discovered 14 drug candidates. The company plans to have one drug candidate entering the clinical stage each year.
The company has established a development platform for novel drugs targeting ActRII pathway. Laekna team has accumulated tremendous experiences and deep knowhow in this specific field and is developing more drug candidates to maximize the value of targeting ActRII receptors.
In addition to LAE102, Laekna has initiated IND-enabling study for LAE103 (an ActRIIB-specific antibody) and targets to advance LAE123 (a dual inhibitor against ActRIIA/IIB) to PCC declaration by the end of 2024. Both of them are our internally discovered antibodies for muscle and other disease indications, which enhance diversities of our ActRII portfolio.
Expected upcoming milestones in 2H 2024
- To achieve primary completion of Single-Ascending Dose part of Phase I clinical trial of LAE102 in the fourth quarter of 2024;
- To advance LAE123 to PCC declaration by the end of 2024;
- To submit IND application for LAE120(USP1 inhibitor) in the fourth quarter of 2024;
- To present more LAE002 (afuresertib)+fulvestrant Phase Ib clinical data and biomarker data as a poster presentation at ESMO in Barcelona, Spain in September 2024;
- To present LAE002 (afuresertib)+Sintilimab+nab-paclitaxel Phase I clinical study results at the 2024 annual global meeting of the International Gynecologic Cancer Society (IGCS) as a poster presentation in Dublin, Ireland in October 2024.
Solid financial positions and continuous commitments in research and development activities
Laekna adopts a prudent funding and treasury policy, aiming to maintain an optimal financial position and to ensure sufficient working capital for our operations and R&D activities.
As at June 30, 2024, our cash and cash equivalents and time deposits were approximately RMB 656.3 million and the net cash used in operating activities during first half of 2024 were approximately RMB 143.3 million.
Conference Call
Laekna will host a conference call at 10:00 am Beijing time on August 19, 2024 (Monday) to discuss the interim financial results.
Join Online: https://s.comein.cn/AHRae
Dial in details:
+86-4001888938 (Chinese Mainland)
+86-01053827720 (Global)
+886-277031747 (Taiwan, China)
+853-68258501 (Macau, China)
+65-31634284 (Singapore)
+1-2025524791 (United States)
+44-2034816288 (UK)
+852-57006913 (Hong Kong, China)
Password: 261809
—End—
Contact Us
Media communication@laekna.com
Corporate and Business Development BD@laekna.com
About Laekna
Stock Code: 2105.HK
Founded in 2016, Laekna is a science-driven, clinical-stage biotechnology company committed to bringing novel therapies to patients with cancer, metabolic diseases and liver fibrosis around the world.
As of June 30, 2024, Laekna has initiated seven clinical trials for LAE102, LAE002(afuresertib), LAE001 and LAE005 to address unmet medical needs in obesity and cancers.
LAE102 is our internally discovered antibody against ActRIIA. It has been shown in the pre-clinical studies to increase lean mass and decrease fat mass. We’ve obtained IND approvals from the FDA and the CDE for LAE102 in obesity indication and are advancing the Phase I clinical trial in China.
Blocking Activin-ActRII pathway could promote muscle regeneration and decrease fat mass. Laekna team has accumulated tremendous experiences and deep know-how in this specific field and is developing more drug candidates (LAE103 and LAE123), in addition to LAE102, to maximize the value of targeting ActRII receptors.
In the oncology area, Laekna has built a comprehensive portfolio of drug candidates, covering the treatment of breast cancer, prostate cancer, ovarian cancer and PD-1/ PD-L1 drug-resistant solid tumors. LAE002 (afuresertib) is a potent AKT inhibitor that inhibits all three AKT isoforms (AKT1, AKT2 and AKT3) as well as one of the only two AKT inhibitors in late-stage development for breast and prostate cancer globally. Laekna has commenced the Phase III clinical trial (AFFIRM-205) for LAE002 in patients with HR+/HER2- breast cancer.
Laekna, Inc. (2105.HK) was listed on the Main Board of The Stock Exchange of Hong Kong Limited (the “Hong Kong Stock Exchange”) on June 29, 2023.
For more information, please visit: https://www.laekna.com/ or https://www.linkedin.com/company/74110713/
Forward-Looking Statements
This press release may contain certain “forward-looking statements” which are not historical facts, but instead are predictions about future events based on Laekna’s current beliefs, assumptions and expectations, commonly identified by words such as "would", "may", "expects", "believes", "plans", "intends", "projects" and other terms with similar meaning. Although we believe that our predictions are reasonable, future events are inherently uncertain and our actual future results or performance may be materially different from what we expect. Accordingly, you are strongly cautioned that reliance on any forward-looking statements is subject to significant known and unknown risks and uncertainties. All forward-looking statements contained herein are qualified by reference to the cautionary statements set forth in this section. All information provided in this press release is as of the date of this press release and are based on assumptions that we believe to be reasonable as of this date, and we do not undertake any obligation to update any forward-looking statement, except as required under applicable law.
-
 2024-11-20Laekna Announces a Clinical Collaboration with Lilly to Develop LAE102, a Novel Monoclonal Antibody Targeting Activin Receptor Type 2A for The Treatment of ObesityMore
2024-11-20Laekna Announces a Clinical Collaboration with Lilly to Develop LAE102, a Novel Monoclonal Antibody Targeting Activin Receptor Type 2A for The Treatment of ObesityMore -
 2024-06-26Laekna Announces First Subject Dosed in Phase I Clinical Trial of LAE102 (a monoclonal antibody against ActRIIA) in China for The Treatment of ObesityMore
2024-06-26Laekna Announces First Subject Dosed in Phase I Clinical Trial of LAE102 (a monoclonal antibody against ActRIIA) in China for The Treatment of ObesityMore

Follow us on Linkedin







